Research Highlights
A novel "Kabuto-like" nickel catalyst forms bioactive frameworks from low-cost phenol derivatives
Abstract:
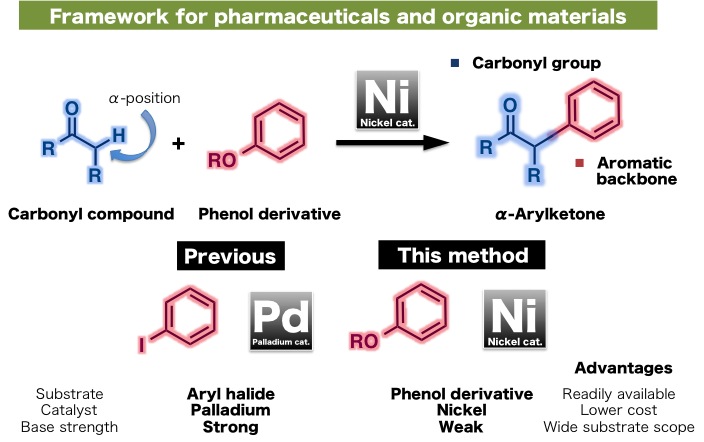
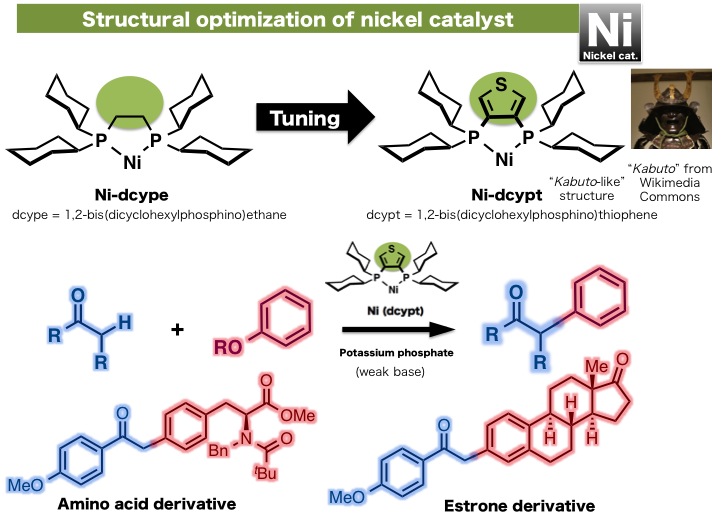
Researchers at ITbM, Nagoya University developed a new nickel catalyst with a "Kabuto-like" structure that was found to catalyze the cross-coupling reaction between carbonyl compounds and readily available phenol derivatives, to form α-arylketones, which are found in many biologically active compounds (Kabuto = a helmet worn by Japanese samurai).
Journal Information:
"Nickel-Catalyzed α-Arylation of Ketones with Phenol Derivatives" Ryosuke Takise, Kei Muto, Junichiro Yamaguchi and Kenichiro Itami, Angewandte Chemie International Edition (2014)
Links:
- Press Release
- EurekAlert! "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- Phys.Org "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- Science Codex "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- Science Newsline "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- Science Daily "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- BBC News "A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives" (May 27, 2014)
- A new 'Kabuto-like' nickel catalyst forms bioactive frameworks from phenol derivatives featured in Bio-Medicine, e! Science News, Press News, Chemistry 2011.org (May 27, 2014), Chemistry Times (May 29, 2014)
- R&D Magazine "New nickel catalyst forms bioactive frameworks from low-cost phenol derivatives" (May 27, 2014)
- Medindia "'Kabuto-Like' Nickel Catalyst Forms Bioactive Frameworks from Phenol Derivatives" (May 27, 2014)
- ResearchSEA "A novel "Kabuto-like" nickel catalyst forms bioactive frameworks from low-cost phenol derivatives" (May 27, 2014)
- Innovations Report "A novel "Kabuto-like" nickel catalyst forms bioactive frameworks from low-cost phenol derivatives" (May 28, 2014)
- Chemistry Views "A Catalyst Worthy of a Samurai" (June 11, 2014)
- Chemistry Processing "Nickel Catalyst Cuts Costs and Waste Issues" (July 25, 2014)

Professor Kenichiro Itami: http://www.itbm.nagoya-u.ac.jp/en_backup/members/k-itami/
Prof. Itami Associate Prof. Yamaguchi


Media Coverage and Related Links:
2014-05-27

