Research Highlights
A new synthesis method for three-dimensional nanocarbons: Connecting carbon by catalysis to create octagonal structures
The Nagoya University Institute of Transformative Bio-Molecules (WPI-ITbM) research group of Kenichiro Itami (Professor and Director, Graduate School of Science, Nagoya University Institute of Transformative Bio-Molecules; research supervisor, ERATO Itami Molecular Nanocarbon Project), collaborating researcher Kei Murakami (Associate Professor, Nagoya University Institute of Transformative Bio-Molecules) and graduate student Satoshi Matsubara (Nagoya University Graduate School of Science) have developed a new catalytic reaction for connecting benzene rings to create an eight-membered ring1) structure, and established a precise and practical method of synthesis for three-dimensional nanocarbons.
Three-dimensional nanocarbons, predicted to have superior physical characteristics including a hardness beyond that of diamonds, are expected to find applications as fuel cell materials and in next-generation organic electronics. However, it has thus far been extremely challenging to synthesize three-dimensional nanocarbons with an accurately controlled structure. Currently known nanocarbon molecules are mostly two-dimensional structures, and thanks to the difficulty of their synthesis, little is understood about three-dimensional nanocarbons.
The key to this method is a new reaction using a palladium catalyst which enables the formation of the octagonal structure. In this reaction, polycyclic aromatic hydrocarbon substrates connect to construct the octagonal structure and enable successful three-dimensional nanocarbon molecule synthesis.
The success of this research represents a revolutionary achievement in three-dimensional nanocarbon molecule synthesis and is expected to lead to the discovery and elucidation of further novel properties and the development of next-generation functional materials. The results of this research were published in the online edition of British journal Nature Catalysis on 7/27/2020 at 11 AM EST.
This research was carried out with the support of the Nagoya University Institute of Transformative Bio-Molecules (WPI-ITbM), part of the World Premier Institute (WPI) program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
It was also supported in part by:
- MEXT grants-in-aid for scientific research for young scientists (A), basic research (B) and challenging research.
- Shorai Foundation for Science and Technology
- Noguchi-Shitagau Research Grant from the Noguchi Institute
About the research
Nanocarbons constructed purely from carbon atoms have garnered a great deal of attention as superior functional molecules with a variety of different properties. These include the fullerene (a sphere, recipient of the 1996 Nobel Prize), the carbon nanotube (a cylinder, discovered in 1991) and graphene (a sheet, recipient of the 2010 Nobel Prize) (Figure 1A). Since Mackay et al. put forward their theory in 1991, a variety of periodic three-dimensional nanocarbons have been proposed. These periodic three-dimensional nanocarbons are predicted to possess superior properties such as a hardness greater than diamonds and spontaneous magnetization, and a great deal of effort has been spent over the years attempting to synthesize these extraordinarily difficult to create theoretical molecules.
One of these challenges is the eight-membered ring structure. In periodic three-dimensional nanocarbons, the eight-membered ring structure appears periodically (Figure 1B). Thus, when synthesizing periodic three-dimensional nanocarbons, consecutive construction of the eight-membered rings is necessary. However, just the construction of the eight-membered ring structure itself is difficult, and it was considered exceptionally challenging to produce multiple eight-membered rings at an efficient rate (Figure 2). If the polycyclic aromatic hydrocarbons that make up the periodic three-dimensional nanocarbon can be connected and the eight-membered rings constructed, this opens up the path to three-dimensional nanocarbon synthesis, representing an innovative method to realize the development of new functional material through periodic construction of eight-membered rings. For these reasons, the development of an efficient method of constructing eight-member rings by organic synthesis was desired.
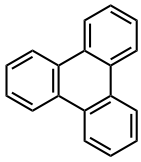
The research group developed a new method for connecting polycyclic aromatic hydrocarbons (PAHs) using a palladium catalyst to produce eight-membered rings (Figure 3A). This method uses a molecule containing chlorine at the PAH bay region2) (a four-carbon unit) as a substrate, with annulative coupling producing the necessary 8 carbons (4 + 4) to form the eight-membered ring. The synthesized molecule is a highly curved (three dimensional) structure based on the eight-membered ring. To put it scientifically, by carrying out a cross-coupling reaction3) with a molecule called biphenylene as the four-carbon unit, they achieved the first ever eight-membered ring structure cross-coupling reaction. The generality of this reaction is high, meaning that it can be used to efficiently construct a variety of three-dimensional molecules. To give a specific example, it was possible to construct a new three-dimensional nanocarbon molecule with three eight-membered rings through introducing a biphenylene molecule to each of the three chlorine areas of triphenylene4) (Figure 3C). This molecule is part of the substructure of a cyclic three-dimensional nanocarbon, and with it being the result of the simultaneous construction of multiple eight-membered ring structures, it is highly innovative.
The synthesized molecule is shown in Figure 4. Based on the eight-membered ring, it takes on a highly curved form, making a new molecule that was extremely difficult to synthesize using existing methods. The section highlighted in blue in Figure 4C shows the synthesized molecule as part of the substructure of the periodic three-dimensional nanocarbon. Thus, through this new method, the group has revolutionized the synthesis of periodic three-dimensional nanocarbons.
By offering an entirely new synthesis method for three-dimensional nanocarbons, this research represents a great leap forward in organic synthetic chemistry, materials science and catalyst chemistry. Nanocarbons synthesized using this method are expected to have a wide range of uses from super-hard materials to fuel-cells.
Figures
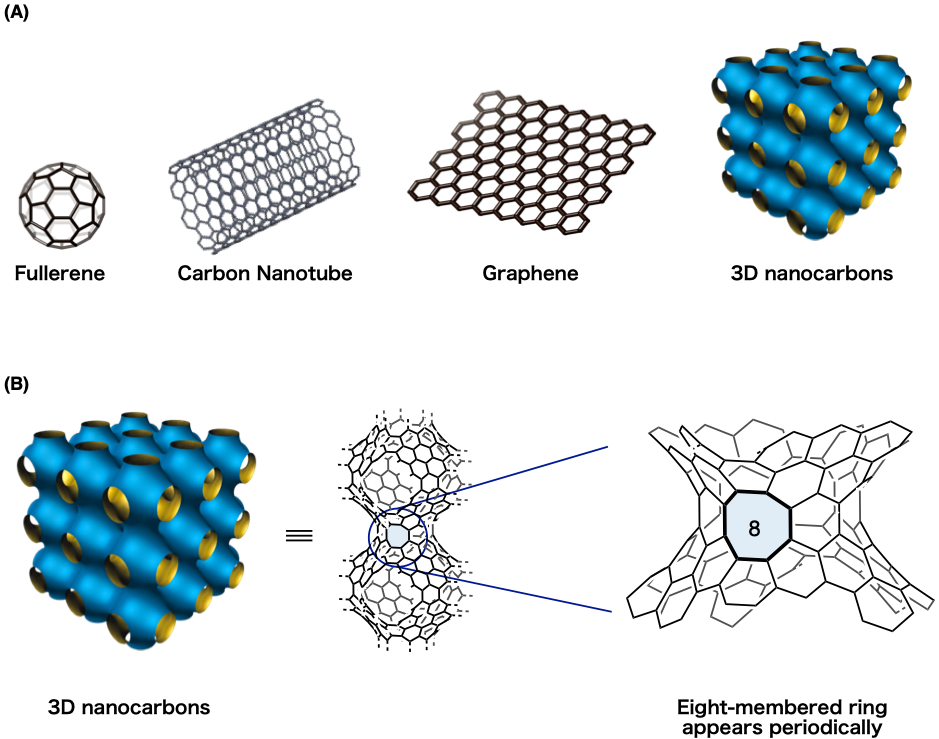
Figure 1: Nanocarbons
(A) A nanocarbon is a material made solely from carbon atoms. They are classified according to the structure formed by those atoms.
(B) Substructure of a three-dimensional nanocarbon in detail. Enlarging the substructure further shows the eight-membered ring.
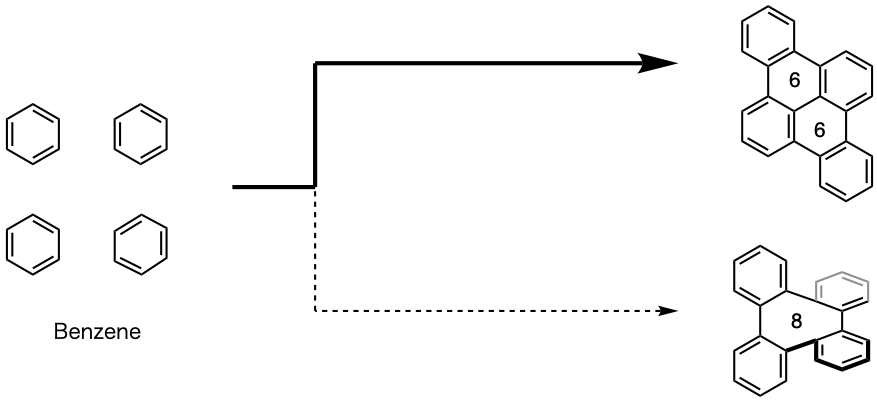
Figure 2: Previous methods for construction using rings
Existing methods for joining benzene rings were primarily designed around six-membered structures. Thus, it was difficult to create curved eight-membered ring structures.
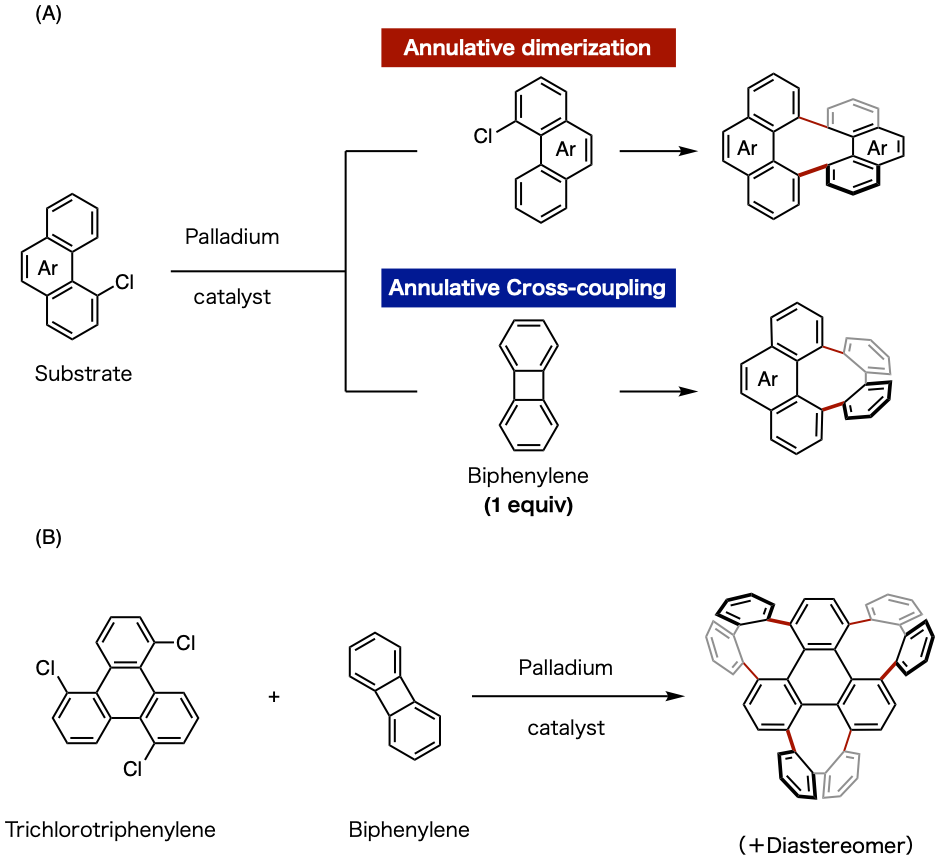
Figure 3: The synthesis reaction used in this research
(A) Annulative dimerization and annulative cross-coupling reaction with palladium catalyst
(B) Three consecutive eight-membered ring construction reactions
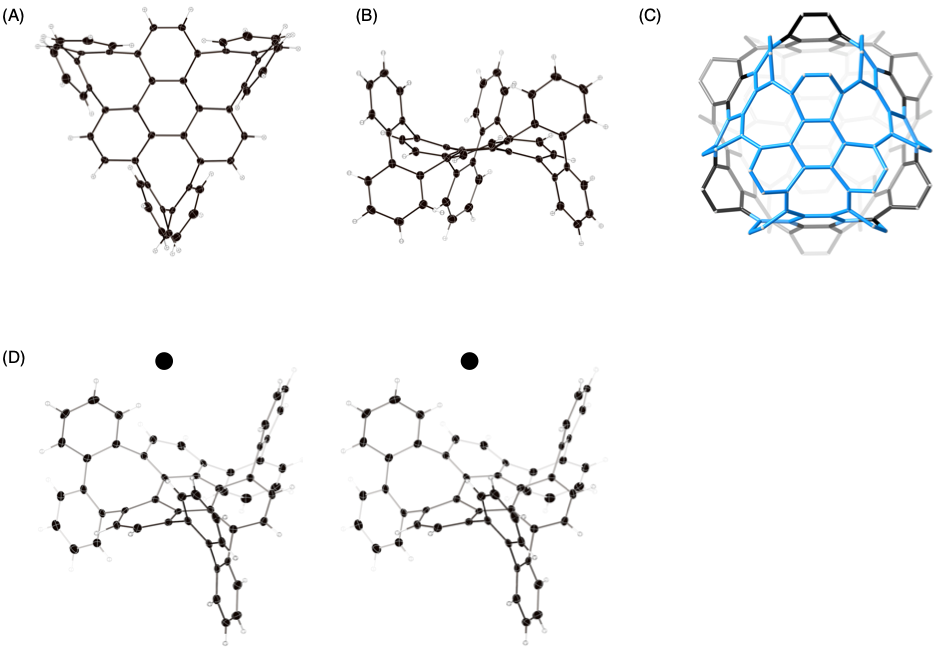
Figure 4: The molecule synthesized in the three consecutive eight-membered ring construction reactions
(A) The structure, shown by X-ray crystallography (front view)
(B) Side view
(C) Substructure of a periodic three-dimensional nanocarbon possessing a similar structure (relevant section highlighted in blue)
(D) Stereoscopic view
Vocabulary
1) Eight-membered ring structure
A ring-shaped structure made from 8 carbons
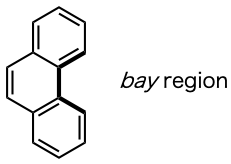
2) Bay region
In polycyclic aromatic hydrocarbons, the name of an area constructed from 4 carbons
3) Cross-coupling
A process in organic chemistry for joining two different molecules
4) Triphenylene
Journal information:
The article "Creation of negatively curved polyaromatics enabled by annulative coupling that forms an eight-membered ring" by Satoshi Matsubara, Yoshito Koga, Yasutomo Segawa, Kei Murakami and Kenichiro Itami is published in Nature Catalysis
DOI:10.1038/s41929-020-0487-0
2020-07-28

