Research Highlights
Establishment of a rapid synthesis method for useful organic fluorine compounds
The Nagoya University Institute of Transformative Bio-Molecules (WPI-ITbM) research team of Professor Cathleen Crudden, Designated Lecturer Masakazu Nambo, JSPS Postdoctoral Fellow Yuki Maekawa and Associate Professor Daisuke Yokogawa of the University of Tokyo have developed a new synthesis method for the efficient production of fluorinated alkenes, an important component in the development of medicines and agrochemicals. This synthesis method's key feature is its ability to synthesize a variety of fluorinated alkenes through the very simple process of adding an organic magnesium reagent, a so called Grignard reagent, a reaction accelerator with organic compounds containing fluorine and elemental sulfur. The research team were able to demonstrate the practical use of this method in shortening the synthesis process of existing bioactive compounds.
This synthesis method, using easy to obtain and prepare reagents, is expected to contribute to the development of new medicines, agrochemicals and organic materials. The results of this research were published on 1 September 2020 in the online edition of the Journal of the American Chemical Society.
This research was supported by the JSPS Grants-in-aid for Scientific Research (No. 17K17805).
About the research
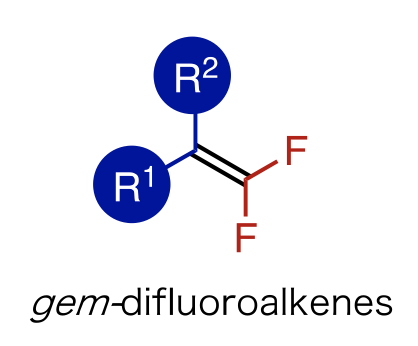
As fluorine is a highly electrically negative element, it is possible to greatly change the properties of an organic molecule by its introduction. Additionally, since bonds between carbon and fluorine are comparatively stronger than those between carbon and hydrogen, it is known to increase stability in metabolism and oxidation. Thus, organic fluorine compounds are found in the majority of agrochemicals and organic materials, with their importance in this field only growing. 'Difluoroalkene' is the generic name for a compound with a structure into which two fluorine atoms have been introduced. These are carbonyl group bioisosteres1), which are also the subject of much attention in pharmaceutical research. Within these, gem-difluoroalkenes, that are all displaced by substituents in double bonds, are a valuable group of compounds that can be used as starting materials for a variety of organic fluorine compounds outside of agrochemicals. Although a number of synthesis methods for difluoroalkenes have previously been developed, these often required the use of dangerously toxic and highly reactive fluoride reagents and were complex and multi-staged. Although methods not requiring hazardous reagents have been reported in recent years, generic synthesis methods for gem-difluoroalkenes have remained severely limited. In the absence of an effective synthesis method, pharmaceutical and materials research that uses this framework has lagged behind and a new method for the straightforward synthesis of gem-difluoroalkenes was desperately needed.
Professor Crudden and Designated Lecturer Nambo's research group have developed a number of free composition methods for useful molecules using sulfones2), a kind of organosulfur compound, as a template. Focusing recently on triflones, a sulfone with a trifluoromethyl group (CF3), they succeeded in the free composition of fluorinated diarylmethanes by way of a cross coupling reaction accompanying flouridation and the severing of the sulfone region.
Thus, the research group aimed to develop a new synthesis method for fluorinated compounds using triflones. That is to say, they thought that by the removal of hydrogen atoms (deprotonation) and activation of carbon-fluorine bonds, followed by desorption of sulfur dioxide (SO2), they could realise an unprecedented transformation reaction to gem-difluoroalkenes. There was no previous example of this kind of reaction, a Ramberg-Bäcklund3) reaction, being used to accomplish sulfone fluoridation.
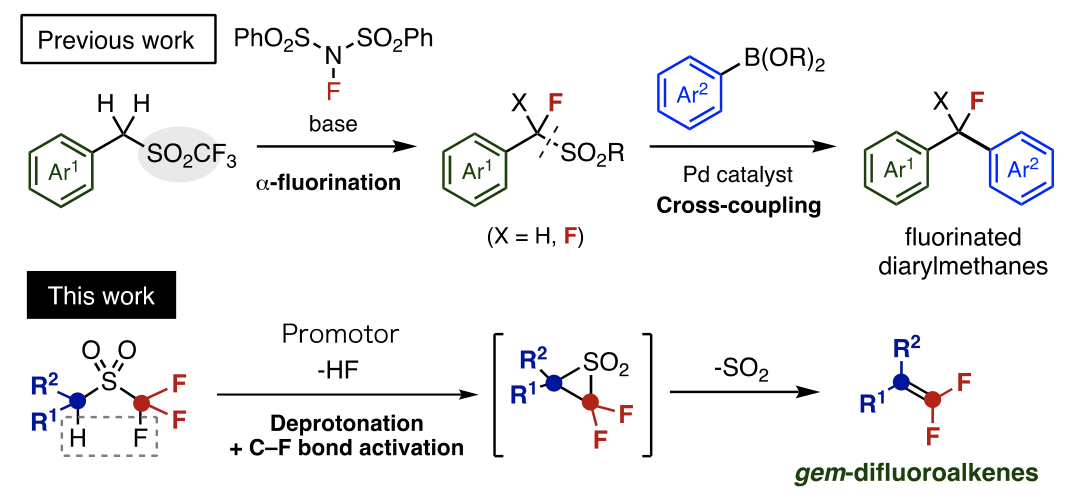
On this occasion, Professor Crudden and Designated Lecturer Nambo's research group succeeded in developing a new synthesis method for gem-difluoroalkenes which reacts triflones with an organic magnesium reagent generalized by organic synthesis. As a variety of carbon substituents can be introduced to the raw material of the triflone, this method greatly speeds up the previously very difficult gem-difluoroalkene synthesis process. In addition to functioning as a base for the extraction of hydrogen atoms, they came to understand that the organic magnesium reagent's function as an activator for carbon-fluorine bonds was particularly important. They demonstrated its exciting characteristics through synthesis experiments and theoretical calculations.
The gem-difluoroalkenes that can be synthesized using this method can be further transformed into a variety of fluorinated compounds. Using this reaction, a fluorinated derivative of the carbonyl group of the antioxidant Zingerone, found in dried ginger root, can be synthesized from cheaply available Eugenol
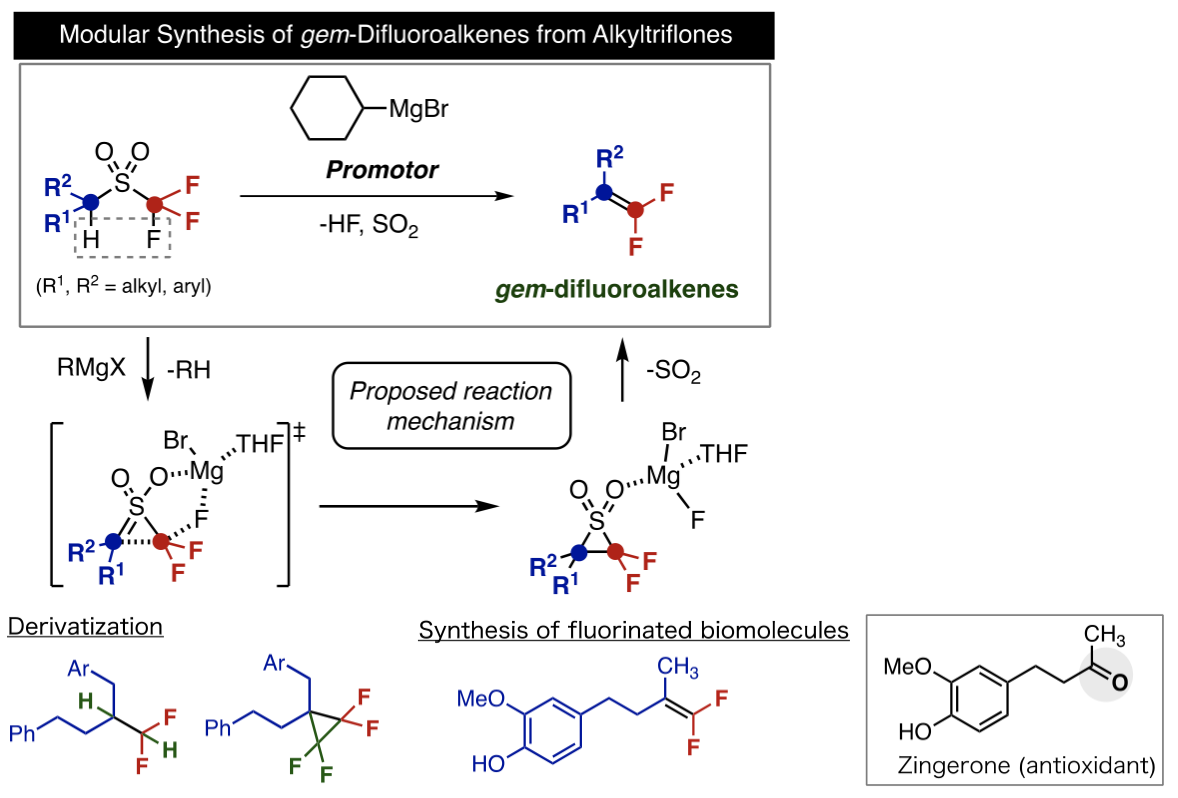
In this piece of research, ITbM's Professor Crudden and Designated Lecturer Nambo successfully developed an effective method for synthesizing gem-difluoroalkenes from triflones. This synthesis method, enabling the synthesis of gem-difluoroalkenes with a variety of structures, presents a new synthesis strategy in organic synthetic chemistry. This synthesis method's ability to use easy to obtain and prepare reagents means that it is expected to find widespread use and contribute to the discovery of new agrochemicals and organic materials.
This research was supported by the JSPS Grants-in-aid for Scientific Research (No. 17K17805).
Vocabulary
1) Bioisostere
In biology, chemical structures with analogous functions. An important concept in recent drug development.
2) Sulfone
An organosulfur compound, a generic name for a molecule with two carbon and two oxygen atoms bonded to organosulfur
3) Ramberg-Bäcklund reaction
When a sulfone is reacted with a base, producing alkenes while emitting sulfur dioxide. Generally, sulfones containing halogens such as sodium, bromine and iodine are used.
Journal information
The article "Alkyltriflones in the Ramberg-Bäcklund Reaction: An Efficient and Modular Synthesis of gem-Difluoroalkenes" by Yuki Maekawa, Masakazu Nambo, Daisuke Yokogawa and Cathleen M. Crudden is published in Journal of the American Chemical Society
DOI: https://doi.org/10.1021/jacs.0c07924
2020-09-02

