Research Highlights
"Zipping-up" rings to make nanographenes ~ A fast and efficient method for graphene nanoribbon synthesis ~
Nanographenes are attracting wide interest from many researchers as a powerful candidate for the next generation of carbon materials due to their unique electric properties. Scientists at Nagoya University have now developed a fast way to form nanographenes in a controlled fashion. This simple and powerful method for nanographene synthesis could help generate a range of novel optoelectronic materials, such as organic electroluminescent displays and solar cells.
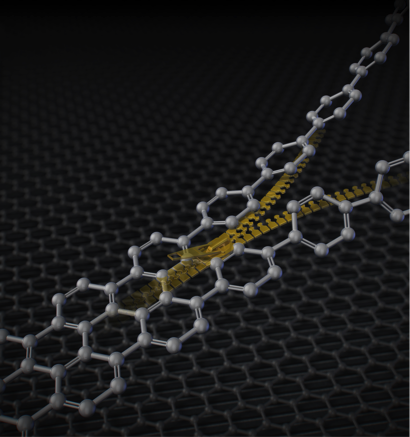 A new method for nanographene synthesis by zipping up partially fused aromatics. Gray colored balls represent carbon atoms and gray colored sticks represent chemical bonds.
A new method for nanographene synthesis by zipping up partially fused aromatics. Gray colored balls represent carbon atoms and gray colored sticks represent chemical bonds.
About the research:
Nagoya, Japan - A group of chemists of the JST-ERATO Itami Molecular Nanocarbon Project and the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University, and their colleagues have developed a simple and powerful method to synthesize nanographenes. This new approach, recently described in the journal Science, is expected to lead to significant progress in organic synthesis, materials science and catalytic chemistry.
Nanographenes, two-dimensional nanometer-wide strips of graphene, are molecules composed of benzene units (Figure 1). Nanographenes are attracting interest as a powerful candidate for next generation materials, including optoelectronic materials, due to their unique electric characteristics. These properties of nanographenes depend mainly on their width, length and edge structures. Thus, efficient methods to access structurally controlled nanographenes is highly desirable.
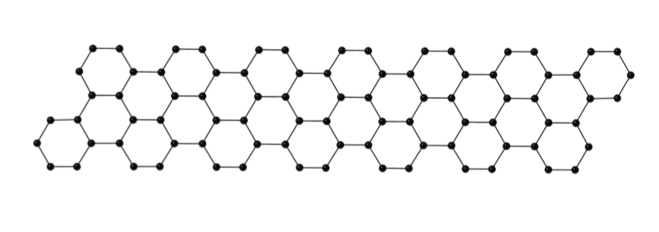 Figure 1. Structure of a nanographene molecule. Benzene (six-membered carbon ring with aromatic properties) units are fused together in one dimension. Balls represent carbon atoms and sticks represent chemical bonds.
Figure 1. Structure of a nanographene molecule. Benzene (six-membered carbon ring with aromatic properties) units are fused together in one dimension. Balls represent carbon atoms and sticks represent chemical bonds.
The ideal synthesis of nanographenes would be a 'LEGO'-like assembly of benzene units to define the exact number and shape of the molecule (upper part of Figure 2). However, this direct approach is currently not possible. The team developed an alternative method that is simple and controls the nanographene structure as it forms in three key steps (lower part of Figure 2).
First, simple benzene derivatives are assembled linearly, through a cross-coupling reaction. Then, these benzene chains are connected to each other by a palladium catalyst that leads to a molecule with three benzene rings bound together in a flat, triangle-like shape. This process repeats all the way up the chain, effectively zipping up the rings together.
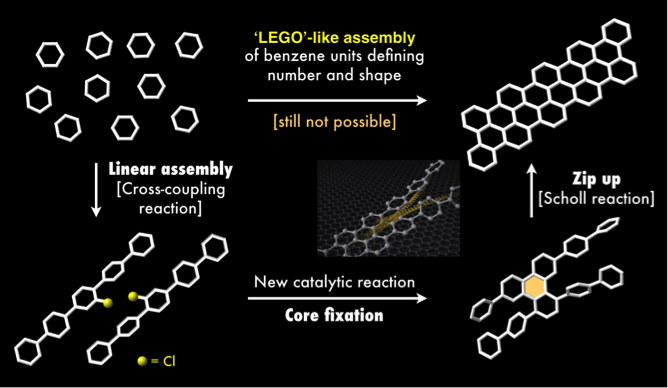 Figure 2. Strategies to synthesize nanographene. A new synthetic approach for nanographenes from simple benzene derivatives via cross-coupling reactions and Scholl reactions.
Figure 2. Strategies to synthesize nanographene. A new synthetic approach for nanographenes from simple benzene derivatives via cross-coupling reactions and Scholl reactions.
The innovation the team developed was a new way to achieve the middle step that forms the three-ring triangle-like unit that forms the core for further reactions to generate the nanographene molecule. A classic technique to connect benzene units uses aryl halides as reaction reagents. Aryl halides are aromatic compounds in which one or more hydrogen atoms bonded to an aromatic ring are replaced by halogen atoms such as fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). This allows benzene to connect at a single point through a process called dimerization, which was discovered by Fritz Ullmann and Jean Bielecki in 1901. However, the Ullmann reaction does not generate nanographenes when using the compound phenylene as the starting material (Figure 3).
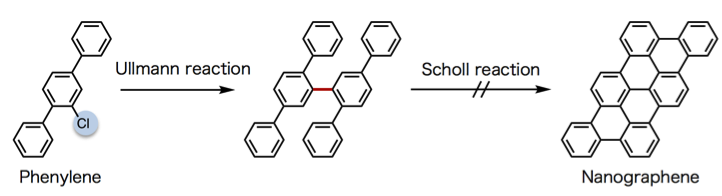 Figure 3. Unsuccessful scheme to synthesize nanographenes. Nanographenes are not generated via the Scholl reaction from the Ullmann reaction product.
Figure 3. Unsuccessful scheme to synthesize nanographenes. Nanographenes are not generated via the Scholl reaction from the Ullmann reaction product.
The team discovered that using a palladium catalyst enabled connections between benzene units at two points, providing the triangle-like structure of three benzene rings (Figure 4a). A triphenylene moiety is formed in the center of each group of rings.
"This discovery was quite accidental," says Designated Associate Professor Kei Murakami, a chemist at Nagoya University and one of the leaders of this study. "We think that this reaction is the key of this new approach for nanographene synthesis."
The team then utilized a process called the Scholl reaction to repeat this process and successfully synthesize a nanographene molecule. The reaction proceeds in a similar manner to benzene rings being zipped up, with the triphenylene moiety acting as the core (Figure 4b).
"One of the most difficult parts of this research was obtaining scientific evidence to prove the structures of the triphenylene derivative and nanographene molecules," says Yoshito Koga, a graduate student who mainly conducted the experiments. "Since no one in our group has ever handled triphenylenes and nanographenes before, I was conducting the research through a 'trial and error' manner. I was extremely excited when I first saw the mass spectrometry signal of the desired molecule to reveal the mass of the molecule through MALDI (Matrix Assisted Laser Desorption/Ionization), which indicated that we had actually succeeded in making nanographene in a controlled fashion."
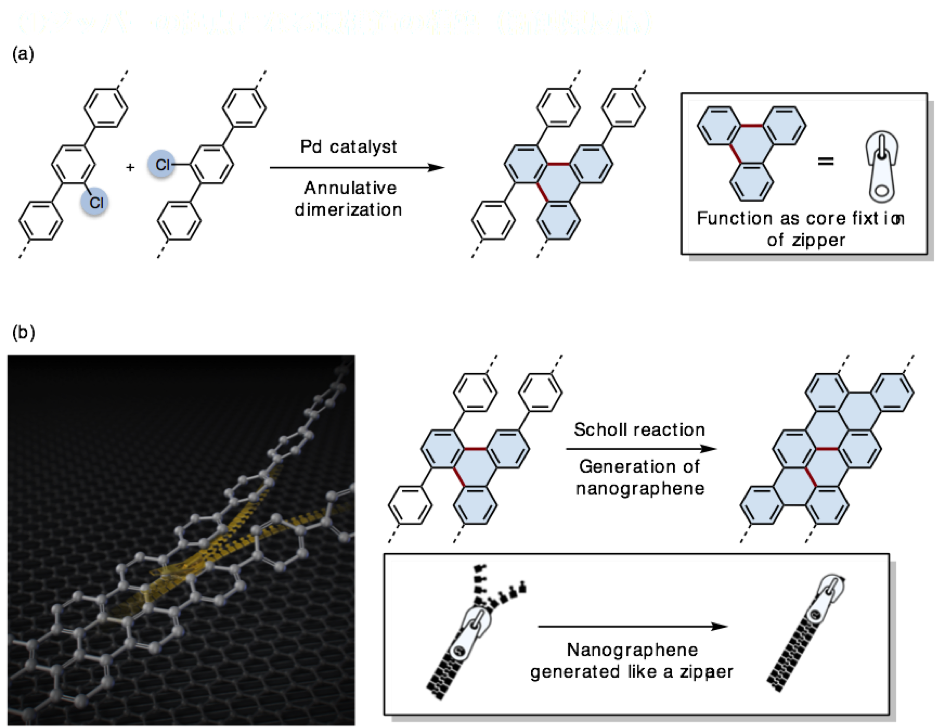 Figure 4. A new synthetic approach towards nanographenes from phenylene: (a) annulative dimerization of phenylene to triphenylene; (b) Scholl reaction "zips up" molecules to make nanographenes.
Figure 4. A new synthetic approach towards nanographenes from phenylene: (a) annulative dimerization of phenylene to triphenylene; (b) Scholl reaction "zips up" molecules to make nanographenes.
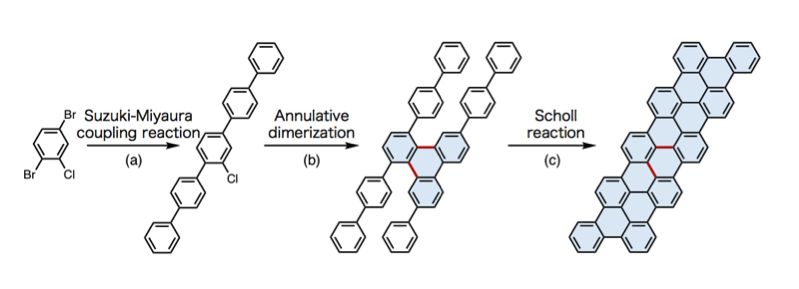 Figure 5. Synthesis of a nanographene through 3 steps from a simple benzene derivative: (a) Suzuki-Miyaura coupling; (b) dimerization reaction (new catalytic reaction); (c) Scholl reaction.
Figure 5. Synthesis of a nanographene through 3 steps from a simple benzene derivative: (a) Suzuki-Miyaura coupling; (b) dimerization reaction (new catalytic reaction); (c) Scholl reaction.
The team had already succeeded in synthesizing various triphenylene derivatives, such as molecules including 10 benzene rings (Figure 6, 1), naphthalene (a fused pair of benzene rings, Figure 6, 2 and 3), nitrogen atoms (Figure 6, 4), and sulfur atoms (Figure 6, 5). These unprecedented triphenylene derivatives could potentially be used in solar cells.
"The approach for creating functional molecules from simple benzene units will be applicable to the synthesis of not only nanographene, but also to various other nanocarbon materials," says Murakami.
"Nanographenes are bound to be useful as future materials," says Professor Kenichiro Itami, director of the JST-ERATO Itami Molecular Nanocarbon Project. "We hope that our discovery will lead to the acceleration of applied research and advance the field of nanographene science."
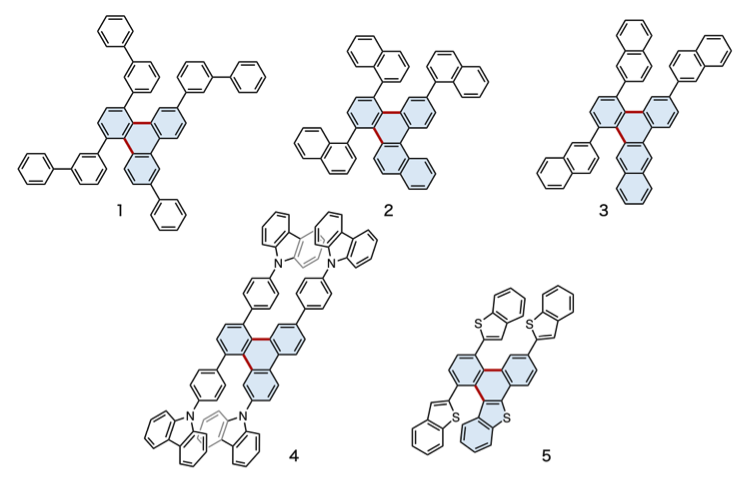 Figure 6. Examples of triphenylene derivatives synthesized by the Itami group's new method. Triphenylene moieties formed in the reaction are colored in blue.
Figure 6. Examples of triphenylene derivatives synthesized by the Itami group's new method. Triphenylene moieties formed in the reaction are colored in blue.
Journal Information
This article "Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization" by Yoshito Koga, Takeshi Kaneda, Yutaro Saito, Kei Murakami, and Kenichiro Itami is published online in Science
Links:



From the left: Prof. Kenichiro Itami, Designated Associate Prof. Kei Murakami, Mr. Yoshito Koga
Media Coverage and Related Links
- C&EN News "Powerful annulation fuses polyaromatics" (February 5, 2018)
- ResearchSEA ""Zipping-up" rings to make nanographenes " (February 8, 2018)
- AlphaGalileo ""Zipping-up" rings to make nanographenes " (February 8, 2018)
- EurekAlert! "'Zipping-up' rings to make nanographenes " (February 8, 2018)
- Science Daily "'Zipping-up' rings to make nanographenes" (February 8, 2018)
- Tech Explorist "'Zipping-up' rings to make nanographenes" (February 8, 2018)
- Nanowerk "'Zipping-up' rings to make nanographenes" (February 8, 2018)
- Phys.Org "A fast and efficient method for graphene nanoribbon synthesis" (February 8, 2018)
- Space Daily "'Zipping-up' rings to make nanographenes" (February 8, 2018)
- Science and Technology Research News ""Zipping-Up" Rings to Make Nanographenes" (February 8, 2018)
- Bright Surf "'Zipping-up' rings to make nanographenes" (February 8, 2018)
- NNNS Chemistry Blog "Introducing annulative chloroaryl dimerization" (January 31, 2018)
- Materials Today "Benzene zips offer controlled way to produce graphene strips" (February 19, 2018)
- Science Magazine " 'Zipping-up' rings to make nanographenes" (February 19, 2018)
2018-02-08

