
Golo Storch
Habilitand and Catalysis Research Center Associate PI, Technical University of Munich (TUM), Germany
Lecture Title
Tailor-Made, Molecular Flavin Catalysts
Abstract
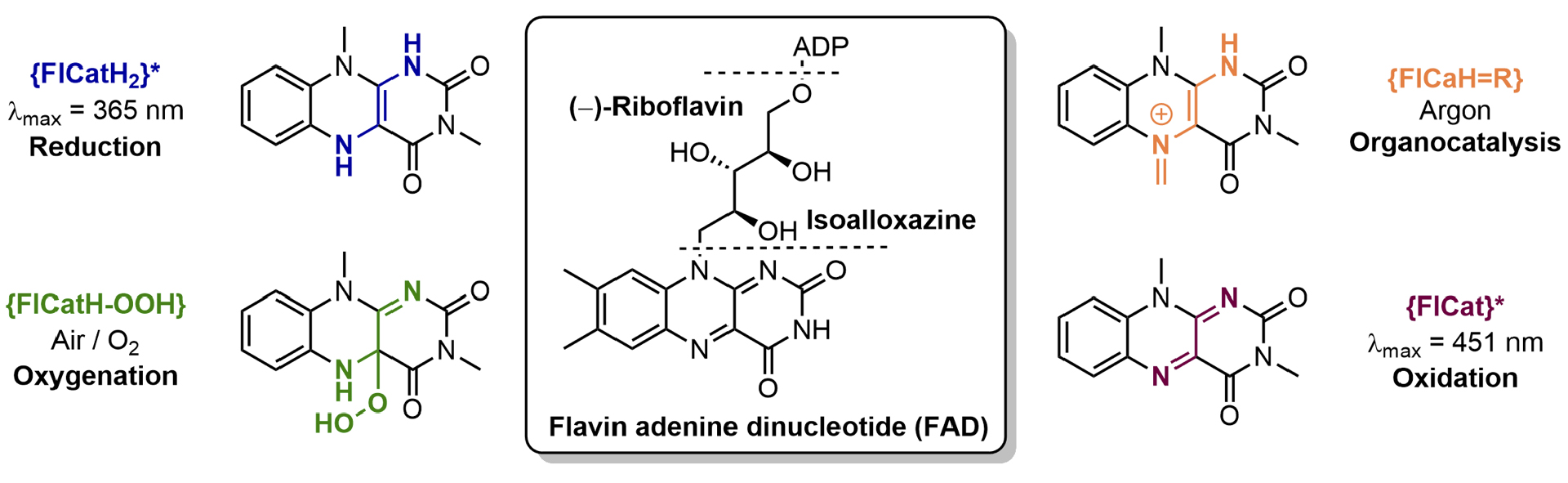
Flavoenzymes are among nature’s most versatile catalysts and mediate reactions ranging from oxidations, oxygenations, ring-contractions, to reductions.[1] However, the astonishingly broad reactivity spectrum of flavoenzymes stands in contrast to the currently limited synthetic use of molecular flavins.
To bridge this gap, we focus on the design and application of molecular flavin catalysts for organic transformations. We are especially interested in sequential reactions since combining various catalytic activities allows for achieving molecular complexity with one single flavin catalyst.
Current examples from our laboratory include desaturation-epoxidation sequences,[2] which were inspired by the enzymatic formation of α,β-epoxyketones, and photochemical catalysis with ring-contracted flavins that rely on both triplet sensitization and H-atom abstraction.[3, 4] We could also develop reductive flavin catalysis with hydroquinoid flavins, which mimics the reactivity of DNA photolyase enzymes.[5]
Reference
- C. T. Walsh and T. A. Wencewicz. "Flavoenzymes: Versatile catalysts in biosynthetic pathways." Natural Product Reports 30, 175-200 (2013). DOI: 10.1039/C2NP20069D.
- A. Walter, W. Eisenreich, and G. Storch. "Photochemical Desaturation and Epoxidation with Oxygen by Sequential Flavin Catalysis." Angewandte Chemie International Edition 62, 42, e202310634 (2023). DOI: 10.1002/anie.202310634
- A. Rehpenn, S. Hindelang, K.-N. Truong, A. Pöthig, and G. Storch. "Enhancing Flavins Photochemical Activity in Hydrogen Atom Abstraction and Triplet Sensitization through Ring-Contraction." Angewandte Chemie International Edition 63, 16, e202318590 (2024). DOI: 10.1002/anie.202318590
- T. Langschwager and G. Storch. Angewandte Chemie International Edition (2024). accepted
- R. Foja, A. Walter, C. Jandl, E. Thyrhaug, J. Hauer, and G. Storch. "Reduced Molecular Flavins as Single-Electron Reductants after Photoexcitation." Journal of the American Chemical Society 144, 11, 4721–4726 (2022). DOI:10.1021/jacs.1c13285