Synthesis of bare aromatic polymers with dendrimer support suggests the creation of unique hybrid materials
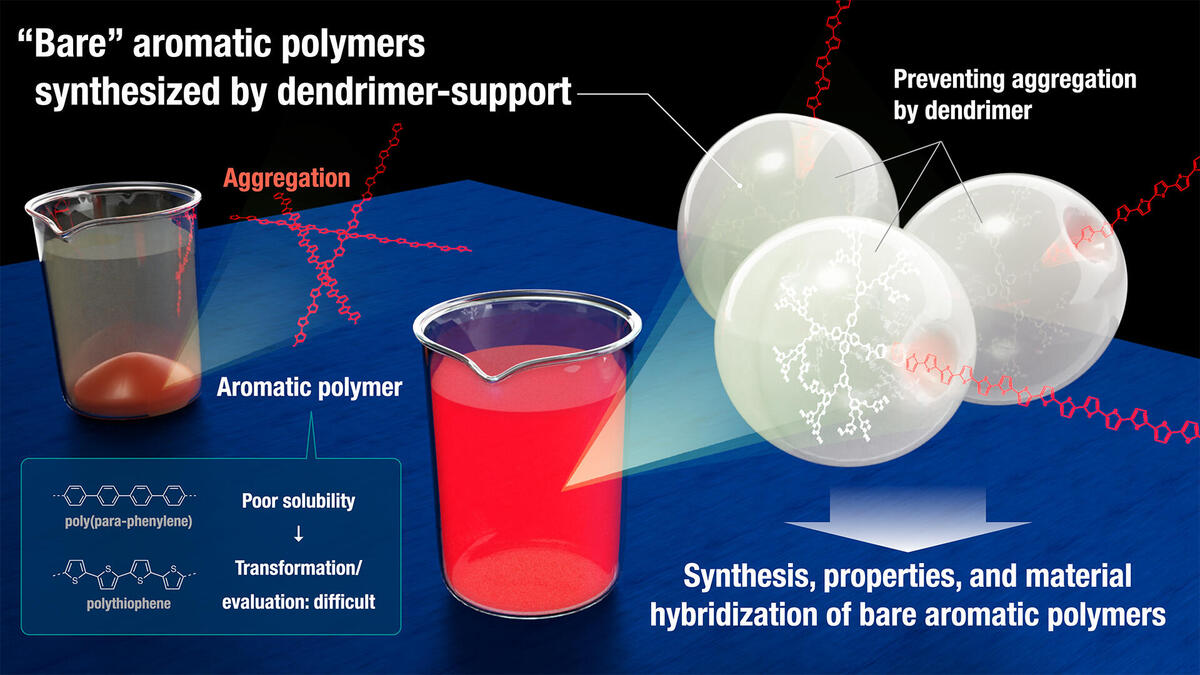
Bare aromatic polymers have the potential to be used in a wide range of high-performance and functional materials. However, their synthesis is hindered by the poor solubility of their parent compounds. Scientists from Nagoya University in Japan have overcome this problem by synthesizing bare aromatic polymers using a dendrimer support. Using the high solubility enabled by dendrimer support, the bare aromatic polymers could be successfully transferred to other materials to create unique hybrids that have potential to become novel functional materials.
Aromatic polymers are the first-choice platform for next-generation materials due to their distinct optical, electronic, and mechanical properties as well as their biocompatibility. In particular, poly(para-phenylene)s (PPPs) and polythiophenes (PTs) have received a tremendous amount of interest due to their high performance as conducting and luminescent materials.
As their properties critically depend on structural factors, there is a lot of interest in the precise synthesis of aromatic polymers. However, due to strong intermolecular π−π interactions, the parent PPPs and PTs are insoluble to solvents without any substituents. Because of this solubility problem, the direct synthesis, property analysis, and material manipulation/transfer of long bare aromatic polymers have remained elusive. Bare PPPs can only be obtained as insoluble aggregates, which are hard to analyze and use.
To solve this problem, a team led by Professor Kenichiro Itami and Designated Associate Professor Akiko Yagi of Institute of Transformative Bio-Molecules at Nagoya University developed a method of hanging aromatic polymers to a dendrimer that enables chain-unsubstituted "bare" aromatic polymers to be handled in solution. Their results were published in Nature Communications.
As dendrimers have a large number of end groups at their periphery, they have a variety of functions. "We assumed that aromatic polymers ligated to a giant dendrimer can gain high solubility owing to the steric hindrance preventing the aggregation." says Shusei Fujiki, the first author of the paper.
The on-dendrimer synthesis of bare aromatic polymers was investigated using a technique called catalyst-transfer Suzuki-Miyaura polymerization. Using this technique, the synthesis of various bare aromatic polymers, such as PTs, PPPs, polyfluorene, and block copolymer, was achieved.
One key finding of their research was that they were also able to unveil the photophysical properties of the π-conjugated backbone of the bare aromatic polymers, which had previously been difficult because of its low solubility. The group also obtained unsubstituted aromatic polymers by releasing the dendrimer support by reduction of the ester group.
Notably, owing to high solubility brought by dendrimer support, the bare aromatic polymers were successfully transferred to other materials, such as silica gel and protein, to create unique hybrids that have organic semiconductor properties and have potential to become novel functional materials. Their proof-of-concept study also showed that the dendrimer-supported aromatic polymer could be utilized as a "reagent" to introduce unsubstituted and insoluble aromatic structures to other materials.
"Poor solubility of aromatic polymers has been a long-standing problem in science. Such aromatic polymers are difficult to access, but that is why they are fascinating. We believe they exhibit intriguing properties derived from bare main chains." says Akiko Yagi, a co-leader of the project.
"This work has opened a new vista in the chemistry of aromatic polymers. The highlight of our research is the transfer of bare aromatic polymers to other materials. A number of hybrid materials with bare π-conjugated structures will become accessible by our strategy." says Kenichiro Itami, leader of the research group. The team hopes to use this technology to make a range of functional hybrid materials.
Funding:
This work was funded by the Japan Science and Technology Agency (JST; JPMJER19R1); the Japan Society for the Promotion of Science (JSPS; JP19H05463, JP19K15537, JP22K14677).
Information
| Title | Synthesis, properties, and material hybridization of bare aromatic polymers enabled by dendrimer support |
|---|---|
| Author | Shusei Fujiki, Kazuma Amaike, Akiko Yagi & Kenichiro Itami |
| Journal | Nature Communications |
| DOI | 10.1038/s41467-022-33100-7 |
| Date | 2022.9 |
